장점
-
전문성
-
신속하고 정확한 결과를 제공
-
타협하지 않는 품질
-
안전성 보장
-
전문가의 건의
-
맞춤형 솔루션
상세내용
We offer more than 40 standarized AAV quality assays.
| Category | SKU# | Test | Method | Timeline(Business Days) |
|---|---|---|---|---|
| Titration | AAVQC001-001 | AAV Genome Titration <ddPCR> | ddPCR | 5 |
| AAVQC001-002 | Custom ddPCR probe and primer synthesis | – | 5-7 | |
| AAVQC001-003 | AAV Capsid Titration | ELISA | 5 | |
| AAVQC001-004 | Infectious Titer <TCID50> | Serial Dilution and qPCR | 21 | |
| Genome Integrity | AAVQC002-001 | AAV Genomic Integrity <CE> | CE | 5 |
| AAVQC002-002 | AAV Genomic Sequencing <TGS> | 3rd Generation Sequencing | 5-10 | |
| AAVQC002-003 | AAV Identity by Sanger sequencing | Sanger sequencing | 3-5 | |
| AAVQC002-004 | Alkaline Gel Electrophoresis | Agarose gels | 6 | |
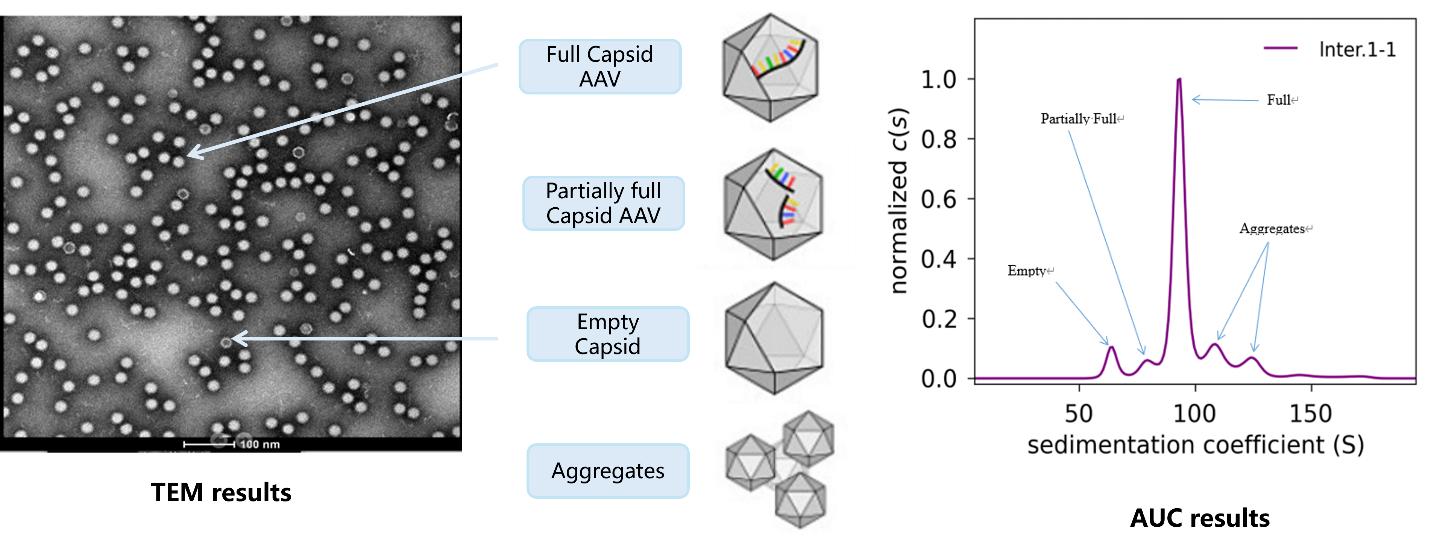
| Capsid characterization, purity and aggregation | AAVQC003-001 | AAV Empty Capsid Rate <AUC> | AUC to analyze empty capsid rate | 5 |
| AAVQC003-002 | TEM (5 pictures) +report | TEM | 3-5 | |
| AAVQC003-003 | DLS (Dynamic light scattering) | Dynamic light scattering | 5 | |
| AAVQC003-004 | AAV Capsid Peptide Mapping | HPLC-MS/MS | 21 | |
| AAVQC003-005 | Capsid Protein Molecular Weight and Ratio | RP-HPLC-MS | 21 | |
| AAVQC003-006 | Capsid purity Analysis <SDS-PAGE> | SDS-PAGE with Silver Staining | 2-3 | |
| AAVQC003-007 | Capsid purity Analysis <SDS-PAGE> | SDS-PAGE with Coomassie Blue Staining | 5 | |
| AAVQC003-008 | Capsid purity Analysis <CE-SDS> | CE-SDS | 5 | |
| AAVQC003-009 | Purity Analysis <AEC-HPLC> | AEC-HPLC | 5 | |
| AAVQC003-010 | Purity and Aggregation Analysis <SEC-HPLC> | SEC-HPLC | 5 | |
| Contamination | AAVQC004-001 | Residual Host HEK293 Cell DNA Quantification | qPCR | 5 |
| AAVQC004-002 | Residual HEK293 Host Cell DNA Sizing | qPCR | 5 | |
| AAVQC004-003 | Residual Plasmid DNA | ddPCR | 5 | |
| AAVQC004-004 | Residual E1A | ddPCR | 5 | |
| AAVQC004-005 | Residual Host Cell Protein | ELISA | 5 | |
| AAVQC004-006 | Residual BSA | ELISA | 5 | |
| AAVQC004-007 | Residual Nuclease | ELISA | 5 | |
| AAVQC004-008 | Residual Affinity Ligands | ELISA | 5 | |
| AAVQC004-009 | Residual PEI | HPLC | 5 | |
| AAVQC004-010 | Residual Tween20 | HPLC | 5 | |
| AAVQC004-011 | Residual Triton X100 | HPLC | 5 | |
| AAVQC004-012 | Residual Iodixanol | HPLC | 5 | |
| AAVQC004-013 | Residual Poloxamer 188 | HPLC | 5 | |
| AAVQC004-015 | Residual Triton Analysis(bundle with endo removal) | HPLC | 5 | |
| Safety | AAVQC005-001 | Endotoxin removal | Triton | 2-3 |
| AAVQC005-002 | Sterility test | Innoculation | 14-20 | |
| AAVQC005-003 | Mycoplasma detection | Gel | 2-3 | |
| AAVQC005-004 | Endotoxin test | LAL | 3 | |
| AAVQC005-005 | Quantitative Endotoxin test | Kinetic Chromogenic Assays | 3 | |
| AAVQC005-006 | Bioburden Test | Plate count method | 5 | |
| AAVQC005-007 | Mycoplasma detection by qPCR | qPCR | 2-3 | |
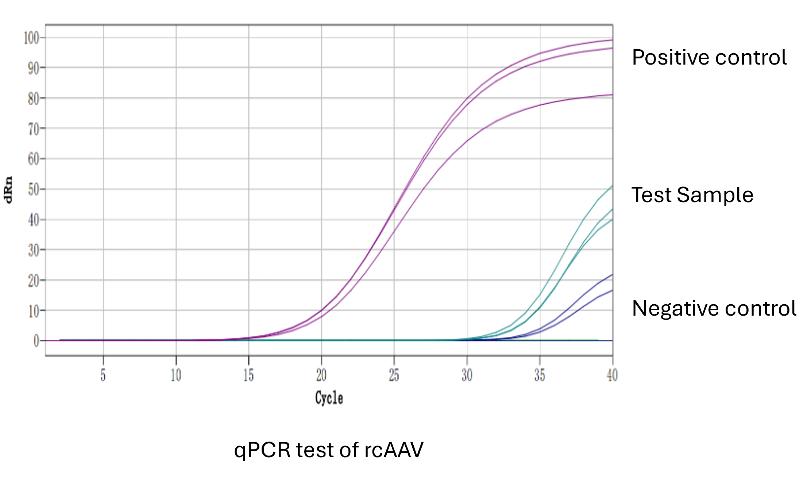
| AAVQC005-008 | Replication Competent AAV (rcAAV) Analysis | Infection on Permissive Cells | 21 |
품질
-
AAV Genome Titer by ddPCR
바이러스의 genome copy 수를 정확하게 측정하여, 정확한 바이러스와 유전자 비례를 보장합니다. 이는 유전자 치료 적용에서 효과적인 용량 결정과 배치 간 일관성 유지에 필수적입니다.
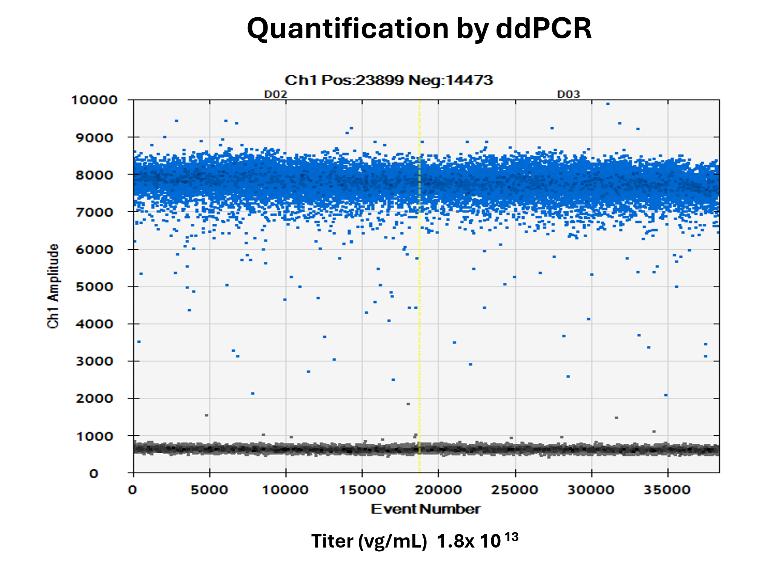
AAV genome titer by ddPCR
-
AAV Genome Integrity by CE
-
AAV Genome Sequencing by Nanopore
-
AAV Empty Capsid Rate Test by AUC
-
Replication Competent AAV (rcAAV) Analysis



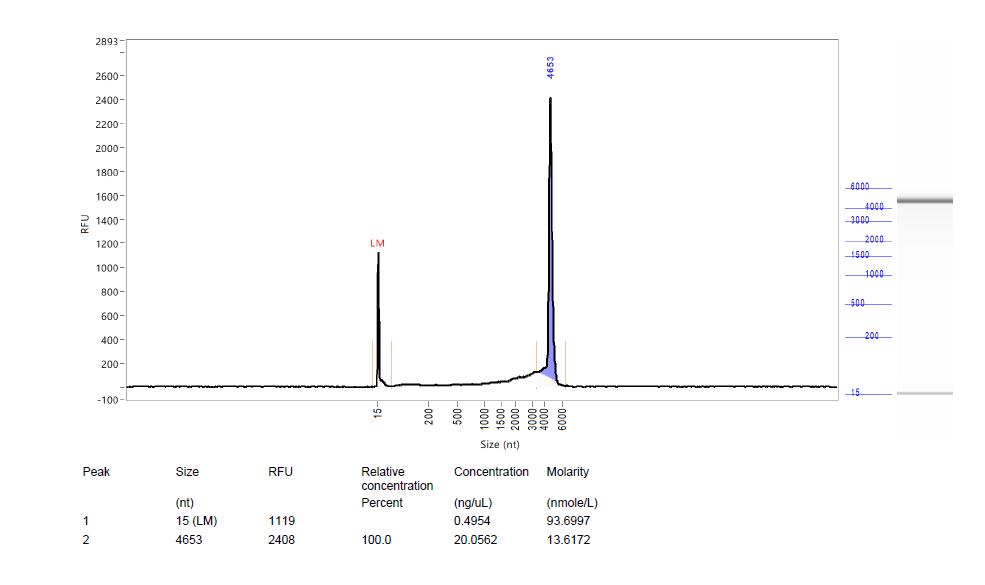
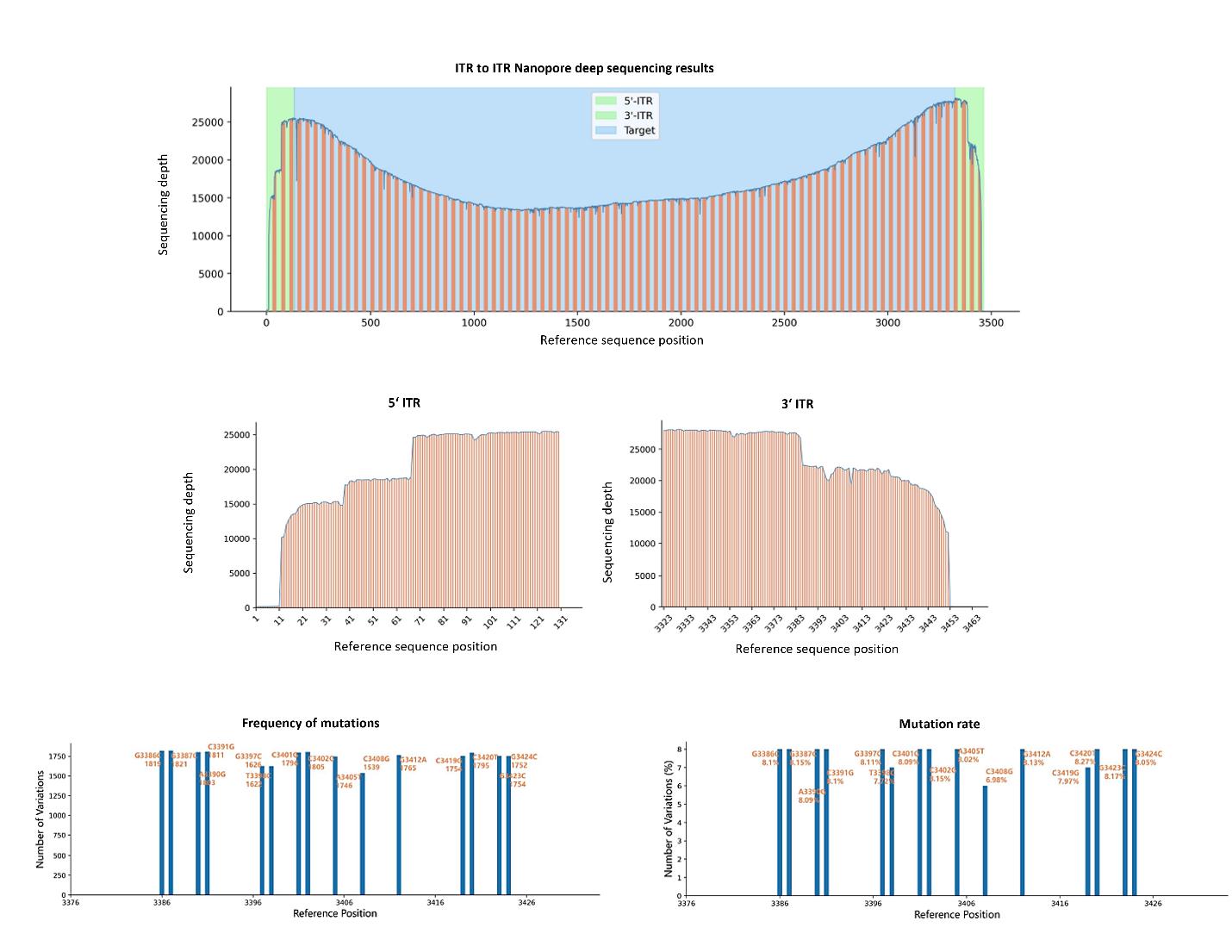 Nanopore sequencing reads depth from 5‘ ITR to 3’ ITR
Nanopore sequencing reads depth from 5‘ ITR to 3’ ITR